PDMonitor®
Last updated on November 27, 2023
The PDMonitor® – developed by pdneurotechnology, is an innovative, non-invasive, continuous monitoring medical device designed to track, record, treat and store a variety of motor and non-motor symptoms frequently occurring in Parkinson’s patients.
Thanks to five lightweight devices to be worn on different parts of the body, a SmarBox (docking station connected to the cloud), where patient data is securely stored, an intuitive mobile application for the patient and an online medical tool facilitate patient-physician interaction.
In June 2019, the PDMonitor® was certified as a Class IIa medical device under Directive 93/42/EEC.
Symptoms monitored:
- Bradykinesia
- Dyskinesia
- Tremor
- Walking disorders
- Postural instability
- ON/OFF conditions
- Activity
- Fluctuations



The prevalence of the disease has doubled over the past 25 years. According to global estimates, more than 8.5 million people had Parkinson’s disease in 2019. (source)
In 2020, around 175,000 people were being treated for Parkinson’s disease in France, with almost 26,000 newly treated. The number of Parkinson’s sufferers will increase by 56% in 2030 compared with 2015, with one in every 120 people over the age of 45 affected by 2030. This increase is mainly due to the aging of the population and the longer duration of the disease. (source)
PDMonitor® - Global architecture

The raw signals collected are processed by machine learning algorithms in the periphery to extract motor symptoms and, together with personalized patient data collected via the patient and caregiver app, are transferred securely to the treating physician’s private cloud, giving him or her a timely, objective and continuous view of the patient’s condition:
A mobile app, which enables patients and caregivers to record information on medication, nutrition and non-motor status, and to obtain medication information and feedback on the patient’s progress
A set of five portable monitoring devices and a Smart Box (edge) where they are mounted when not in use by the patient, to download, process/analyze data, extract symptoms and load,
A private physician cloud where patient information is stored
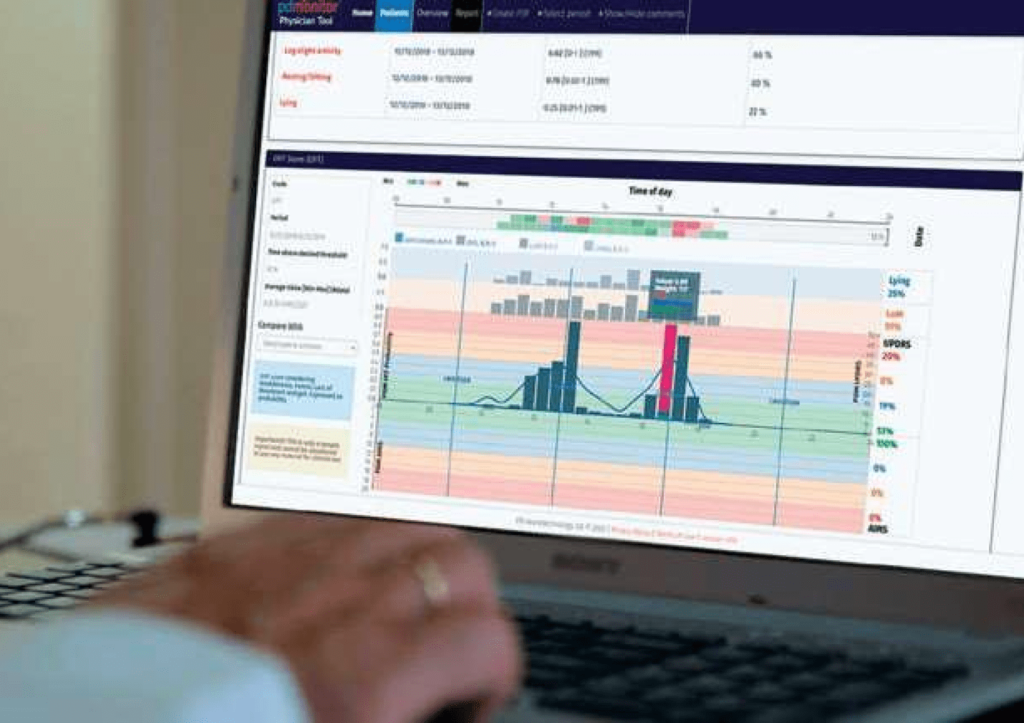
A medical tool, which graphically presents to the doctor the patient grouping, patient condition, patient motor symptoms and patient information, in a quick summary for assessment and detailed information, using daily heat maps and updates every 30 minutes
PDMonitor® - A unique case of Digital Health
Safety / User-friendly / Performance
- CE-marked, Class IIa device
Monitoring functionality based on existing clinical studies
Data / information security
- PD Neurotechnology is ISO 27001 certified, by DQS
Cloud database storage is encrypted and has multiple security measures in compliance with HIPAA and RGPD
Microsoft Azure physical storage is one of the safest and most secure servers meeting the requirements of :- HDS (France)
- HIPAA / HITECH
- HITRUST
- MARS-E
- NEN-7510 (Netherlands)
- NHS IG Toolkit (UK)
Data privacy
RGPD compliant
Interoperability
with patient consent, data can be used by the system and other applications
all scales used are based on UPDRS standards, widely used in PD monitoring
Other quality requirements
ISO 13485 (MDD Annex II) certified by DQS Med Germany
